

Once all water has been boiled to steam, the temperature will continue to rise linearly as heat is added. At the boiling point, temperature no longer rises with heat added because the energy is once again being used to break intermolecular bonds. Once all ice has been melted, the temperature again rises linearly with heat added. At this point, there is a mixture of both ice and water. That heat energy is instead used to break intermolecular bonds and convert ice into water. Then, the heat added does not change the temperature.
Temperature increases linearly with heat, until the melting point. The graph shows the temperature of ice as heat is added. If that amount of energy is added to a mole of that substance at boiling or freezing point, all of it will melt or boil, but the temperature won't change. These amounts of energy are the molar heat of vaporization and molar heat of fusion. To boil or melt one mole of a substance, a certain amount of energy is required. This is because once water reaches the boiling point, extra energy is used to change the state of matter and increase the potential energy instead of the kinetic energy.
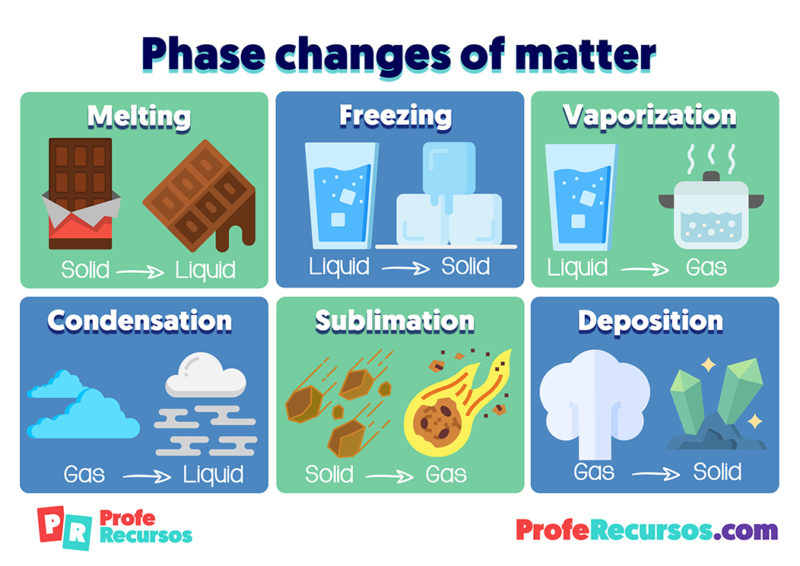
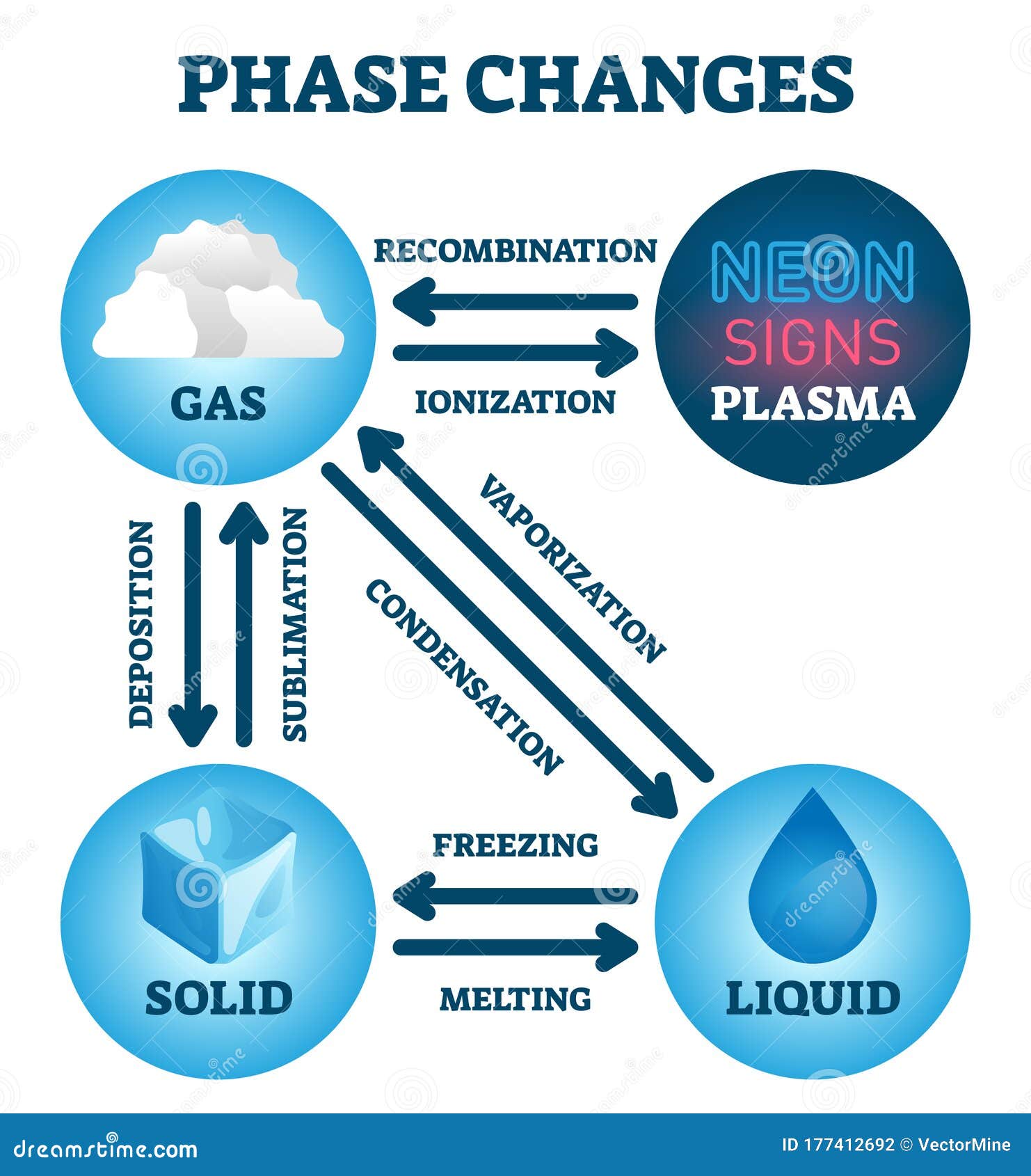
Only after it has completely evaporated will it get any hotter. If you boil water, it never goes above 100 degrees Celsius. The kinetic energy of a molecule is directly proportional to its temperature. It has interesting electrical properties, but it is not important in the scope of General Chemistry.Įnergy Changes Helpful Hint! A plasma is simply a gas that has been completely ionized, so that there is a mixture of positive ions and electrons. The diagram on the right also shows the plasma state of matter. If the temperature and pressure change and move across a line in the diagram, the phase will also to change. Any further, and the characteristics become a blend of liquid and gas. The critical point is the highest pressure and temperature that the three normal phases can exist. Points that are on a line are where two phases of matter can coexist. There are six ways a substance can change between these three phases melting, freezing, evaporating, condensing, sublimination, and deposition (2). The triple point is where all three phases of matter can exist in equilibrium. Soild -> Gas This is called sublimation process ( solid needs to be heated, example leaving dry Ice on the surface, solid dry ice gains heat from surroundings and turns into gas.Phase diagrams predict the phase of a substance at a certain pressure and temperature. Gas -> Liquid This is called condensation process ( gas needs to be cooled, example condensation of steam on the mirror, when one throws steam on mirror or cold surface, steam or gas loses heat to the surface and changes to gas) Liquid -> Gas This is called evaporation process ( liquid needs to be heated, example evaporation of water, water on taking up heat from the flame or from the surroundings changes to gas) Liquid -> Solid This is called freezing process ( liquid needs to be cooled off, example freezing of water, liquid on losing heat to surroundings cools off to form soild.) Soild -> Liquid This is called melting process ( solid needs to be heated, example melting of butter, solid on taking up heat changes to liquid) Adding heat or taking off heat brings in change of state/ phase. Heat plays an important role in converting one state of matter to another.


 0 kommentar(er)
0 kommentar(er)
